Cerdanyola del Vallès, 30th October 2019 Plasmodium falciparum parasite, transmitted through mosquito sting, causes the malaria disease infecting red blood cells of its victim. In the last two decades, the parasite has evolved into drug-resistant strains. "Recently, the increasing geographical spread of the species, as well as resistant strains has concerned the scientific community, and in order to improve antimalarial drugs we need to know how they work precisely", explains Sergey Kapishnikov, from the University of Copenhagen in Denmark, and the Weizmann Institute, in Israel, and leader of the study.
Once inside red blood cells, Plasmodium ingests hemoglobin (the protein in charge of oxygen transport) as a nutrient. After digesting, iron-containing heme molecules are released, which are toxic to the parasite. However, these molecules crystallise into hemozoin, a disposal product formed from the digestion of blood by the parasite that makes the molecules inert. As reported in previous studies, for the parasite to survive, the rate at which the heme molecules are liberated must be slower or the same as the rate of hemozoin crystallization. Otherwise there would be an accumulation of the toxic heme within the parasite.
Quinoline-family drugs, which include quinine-based antimalarial pills, effectively damage the parasite. The scientific community has suspected that the reason for their success is the inability of the heme to crystallise. Until today, all studies of the drug action on heme crystals have been done either on model systems or on dried parasites, which yielded limited data and opened room for speculation. Kapishnikov and his team, which include scientists from Denmark, Spain, Germany, Israel and France, decided to find out the mode of action of established drugs like chloroquine (although they used the analog bromoquine) in fully hydrated, rapidly frozen, Plasmodium falciparum-infected red blood cells. Rapid freezing creates instant snapshots of the living stage of the cells such that chemical distribution therein is unaltered by sample preparation.
Synchrotron complementary techniques
In this case, the same cells maintained under cryogenic conditions had to travel across Europe. The researchers brought them in and out of synchrotron facilities, in order for their structure to be mapped in three dimensions by soft X-ray cryo-tomography at the MISTRAL beamline from the ALBA Synchrotron and BESSY-II in Berlin. This technique, only available in four countries all over the world (UK, USA and these two mentioned in Germany and Spain), is the unique way to image whole cell samples in their native state without any chemical treatment or sectioning.
Finally, cells were brought to ESRF for mapping of bromine and iron distribution by the X-ray fluorescence nano-probe. The synchrotron data were analyzed back in Denmark where scientists determined the correlation between the different imaging modalities and they calculated and interpreted the concentrations of bromoquine at the surface of hemozoin crystals, at the membrane and within the lumen of the parasitic digestive vacuole – the site of the drug action.
The mapped infected red blood cells at the synchrotrons showed that bromoquine caps hemozoin crystals, thereby inhibiting the hemozoin crystal growth and hence, sabotaging heme detoxification. Surprisingly, they also found that bromoquine accumulates in the digestive system of the parasite, which enhances the drug’s efficiency in depriving heme from docking onto the hemozoin crystal surface.
"These results show a model that can be generalized to all quinoline drugs, such as quinine, and our approach can be extended to other families of antimalarial drugs, such as artemisinins", explains Kapishnikov. Malaria remains one of the biggest killers in low-income countries, estimates of the number of deaths each year range from 450,000 to 720,000, with the majority of deaths happening in Africa. "We hope that this knowledge will let us go a step further in designing new, effective drugs against resistant malaria strains", he concludes.
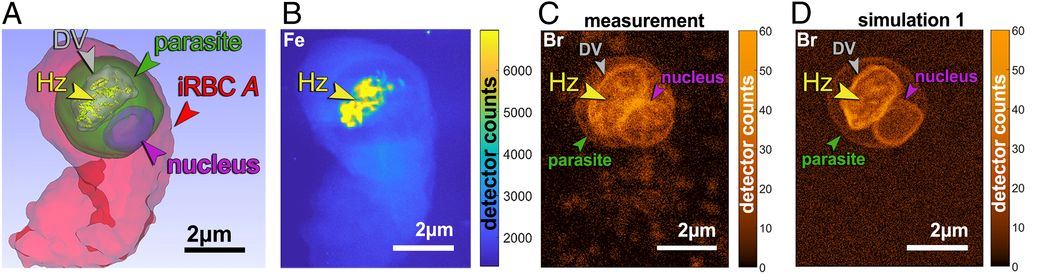
Surface rendering, measured and simulated X-ray fluorescence maps of a bromoquine-treated infected red blood cell (iRBC) labeled A. (A) Surface rendering of a soft X-ray tomography segmentation. (B) Measured iron (Fe) X-ray fluorescence map.(C) Measured brome (Br) X-ray fluorescence map. (D) Simulated Br X-ray fluorescence map. Br atoms were evenly distributed over the surface of the digestive vacuole (DV) membrane, the parasite nucleus, and the parasite membrane with the density of 5·10^3 atoms per μm2, and on the surface of hemozoin (Hz) crystals with a density corresponding to 10% bromoquine surface coverage.
Reference:




