Cerdanyola del Vallès, 4th April 2019. Since it was first produced by playing with a sticky tape in a lab, graphene has had a revolutionary impact on material science, as well as fundamental and applied physics. This bidimensional material, a conductor of one atomic carbon layer with an extremely high electron mobility and mechanical strength, has allowed the study of macroscopic quantum effects and, as experts have speculated, it may expedite a new generation of fast and small electronic devices.
The work, recently published in the journal 2D Materials, was led by a team of the Institute of Materials Science of Madrid (ICMM-CSIC) in collaboration with the ALBA Synchroton, the Donostia International Physics Center (DIPC) and the Centro de Física de Materiales (CSIC/UPV-EHU). It targets the electronic properties of graphene, which, although highly promising, still have to be controlled in a more efficient way in order for it to be considered as a new material in the semiconductor industry.
A simple path to Na intercalation under graphene has been demonstrated, which is also reversible and allows for the recovery of the graphene, being one step closer to the foreseen atomically thin carbon layer that may substitute silicon in future electronic devices. In addition, "this methodology could be extrapolated to other alkali metals, and foster interesting studies in advanced batteries", says Irene Palacio, researcher from ICMM-CSIC.
From the kitchen to the high-tech materials science lab
Experiments were carried out at ALBA using the photoemission electron microscope (PEEM) branch of the CIRCE beamline and started from graphene layers prepared onto a metallic iridium substrate. Even though the initial interaction of graphene with iridium is weaker than with other substrates, it is still enough to degrade its electronic properties. A thin NaCl overlayer was deposited onto the graphene in order to test it as a potential cheap and easy-to-remove protective layer against ambient conditions. However, when NaCl is exposed to synchrotron light, the chlorine desorbs while sodium intercalates under graphene, decoupling it from the iridium substrate and creating an effective n-doping, i.e., transforming it into a semiconductor.
The intercalation process was structurally monitored by electron diffraction (LEED) and chemically by photoelectron spectroscopy. With the former (Fig. 1), it was observed that the moiré superstructure, created by the interaction of graphene with iridium, disappeared.
With the latter (Fig. 2), the transition of sodium from a salt crystal to metallic sodium, i.e. from NaCl to Na, was observed.
Finally, by means of angular resolved photoemission (ARPES), the modification of the graphene electronic structure due to decoupling from the substrate, i.e., its n-doping, was ascertained (Fig. 3).
The experiments also included a final annealing process, at 823 K, which allowed for the recovery of the initial graphene, without any damage and again coupled to the iridium substrate.
The PEEM microscope at ALBA's CIRCE beamline "offered the perfect combination of several surface characterization techniques needed for this study", according to Michael Foerster, beamline scientist at CIRCE. "This is an interesting example where synchrotron radiation is more than just a passive probe, but also provides the trigger for the photodissociation of the NaCl", continues Michael Foerster.
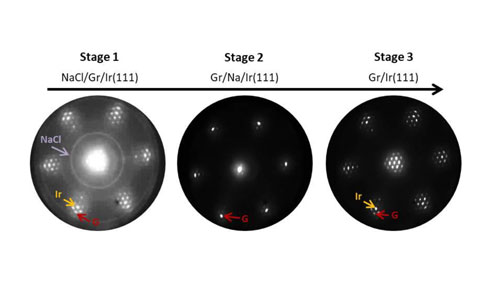
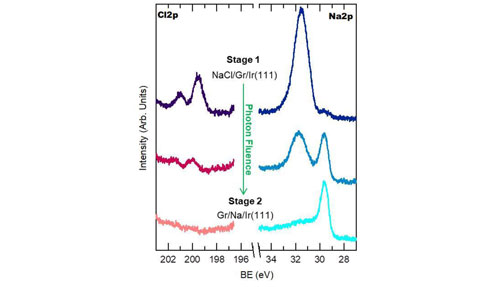
At the left, Fig. 1: Electron diffraction patterns during photo-dissociation of NaCl and the intercalation of Na, from NaCl/Gr/Ir(111) -> Gr/Na/Ir(111) -> Gr/Ir(111). At the right, Fig. 2: Evolution of Cl 2p and Na 2p core levels of a NaCl/Gr/Ir(111) sample during XPS measurement as a consequence of the photon irradiation.
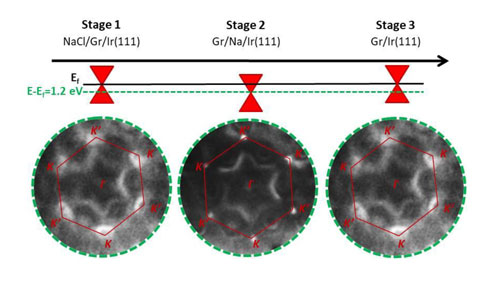
Fig. 3: Constant energy ARPES maps showing the evolution of the electronic structure of the NaCl/Gr/Ir(111) system when irradiated with synchrotron radiation.




