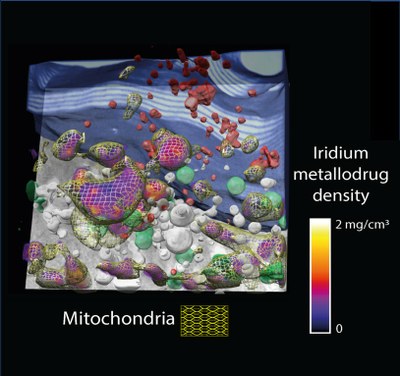
Real data showing a 3D section of a cryopreserved breast cancer cell. The purple-to-yellow palette indicates iridium density, and the mitochondria are shown as a yellow mesh. The iridium can be exclusively observed inside the mitochondria. Other organelles are present: nucleus in blue, dense vesicles in red, lipid droplets in green and vacuoles in white.
Cerdanyola del Vallès, 26th February 2020. Chemotherapeutics are key players in the clinical setting to fight most types of cancer, and novel chemicals hold the promise to facilitate new and unique intracellular interactions that modulate the cell machinery and destroy the tumour cells. Equally necessary are new tools that enable the unequivocal location and quantification of such molecules in the intracellular nano-space, so that their therapeutic action is fully understood.
Researchers from IMDEA Nanociencia, the ALBA Synchrotron, the European Synchrotron Radiation Facility (ESRF) and the National Centre for Biotechnology (CNB) have developed a new family of organo-iridium drug candidates about a hundred times more potent than the clinically used drug cisplatin.
In order to understand the therapeutic potential of the compound, it is mandatory to accurately localize its fate within the cell ultrastructure with minimal perturbation. To this aim researchers have correlated on the same cell, for the first time, two 3D X-ray imaging techniques with a resolution of tenths of nanometers: cryo soft X-ray tomography, at MISTRAL beamline at ALBA Synchrotron, and cryo X-ray fluorescence tomography, at ID16A beamline at ESRF. These techniques help elucidate the 3D architecture of the whole cell and to reveal the intracellular location of different atomic elements, respectively.
Thanks to this study, recently published in the journal Angewandte Chemie International Edition, scientists have discovered that the mechanism of therapeutic action of this family of iridium-based anticancer agents appears to be radically different to that of cisplatin’s, which is crucial to circumvent drug resistance.
Dr. Ana Pizarro, research scientist at IMDEA Nanociencia, explains: “We have been able to observe - using highly advanced synchrotron cryo-techniques - our extremely potent iridium drug candidate in cryopreserved breast cancer cells with nanoscale resolution. This means that we have been able to unambiguously locate iridium in the cancer cell mitochondria and, most strikingly, nowhere else”. Mitochondria are important organelles orchestrating the cell fate since they are key determinants of regulated cell death induction. It is important that the drug has been exclusively located in the mitochondria as this can help minimise off-target interactions, which usually lead to the undesirable side effects that chemotherapy triggers in cancer patients.
Dr. Javier Conesa, key investigator in this project working at MISTRAL beamline in ALBA at the time this research was carried out, adds: “We have also been able to quantify the iridium drug specifically inside the mitochondria, which is also very important and unique, and not possible when tagging with fluorophores or with bulk experiments. Also, it is important to notice that the detection was performed using whole cells, without any sectioning, and that allows resolving the whole cell context, and in cryo conditions which means that the cell structure and chemical composition are closely preserved to the native conditions. This newly developed 3D correlative technology can also be applied to other biological problems and hence we expect to study the distribution of other interesting elements”.
Pizarro adds: “These compounds have the potential of being extremely effective against cancer, yet unless we fully understand their complete journey within the tumour cell, they don’t stand a chance to enter the drug development pipeline. Not only will this understanding help advance truly novel chemotherapeutics in the clinic, but it will also provide innovative tools to intervene processes related to cancer progression. It is a long road and this work represents the first step”.
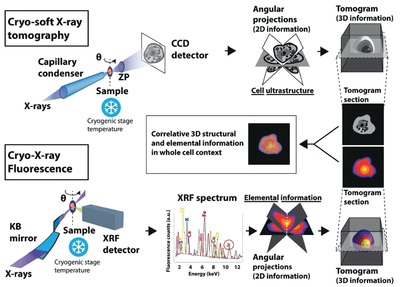
Correlative approach between cryo soft X-ray tomography (cryo-SXT) and cryo hard X-ray fluorescence (cryo-XRF). Samples measured by cryo-SXT to obtain the 3D ultrastructure of the cell were also analysed by cryo-XRF to measure the 3D distribution of multiple elements of interest. The information obtained using both techniques was correlated to provide 3D elemental information in the whole cell context in close-to-native conditions.
Dr. Javier Conesa, after a postdoc at ALBA synchrotron, joined the National Centre of Biotechnology to implement a cryocorrelative microscopy platform.
Dr. Ana Pizarro is a research scientist at IMDEA Nanociencia with a Ramón y Cajal Fellowship. Where she studies how metal-based compounds can modulate the cancer cell machinery.
Link to IMDEA Nanociencia news: https://www.imdeananociencia.org/home-en/news/item/nanoimaging-the-intracellular-space-to-aid-drug-development




