Cerdanyola del Vallès, 28th February 2019 The researchers from IBV, in collaboration with the University of Glasgow, have revealed the molecular mechanism that regulates decision-making processes in the life cycle of certain bacteriophages. The study is published in the journal Molecular Cell and could have future applications in the field of Biotechnology.
Bacteriophages, also known as phages, are viruses that exclusively infect bacteria and are considered the most abundant organisms on the planet. Phages can present lytic or lysogenic life cycles. In the lytic cycle, once its genome is injected into the bacterial host, the phage reproduces by parasitizing the molecular machinery of the bacteria and, finally, breaks the membrane of the cell while releasing its progeny. On the other side, in a lysogenic cycle, the genome injected by the phage to its host is inserted into the bacterial DNA and remains there as a prophage, replicating as part of the bacterial genome and, under the signal of a specific activator, activates its lytic cycle resulting in a bacteria breaking to spread the new phage’s generation.
Alberto Marina, researcher at the IBV, explains that "while some phages only have a lytic cycle, others can alternate between this cycle and the lysogenic. However, our understanding of the molecular mechanism that underpins this decision once the phage has infected the bacteria is very limited."
Scientists have investigated a new decision-making system that has recently been discovered in phages that infect bacteria of the genus Bacillus, called as 'arbitrium'. "Communication through ‘arbitrium’ depends on the production and secretion of a hexapeptide called AimP during the lytic cycle. Therefore, the level of AimP in the medium is an indicator of the amount of active phages. Phages can measure these levels thanks to the AimR protein, a transcription factor that is regulated by AimP and controls the expression of the negative regulator of lysogeny. In this way, the phage can decide between the lytic or the lysogenic cycle depending on the number of competing phages and, consequently, on the possibility of finding a free host", adds Marina.
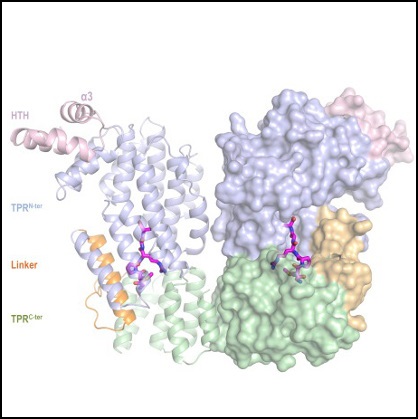
"The key point of our work has been to unveil the crystalline structures of the AimR protein from the phage of Bacillus subtilis, SPbeta. This structural information has been obtained thanks to X-rays diffraction technique in the XALOC beamline at the ALBA Synchrotron and has helped us to understand the molecular basis of the ‘arbitrium’ communication mechanism", concludes Francisca Gallego del Sol, from the IBV. The work provides important information on this new regulatory pathway and reveals clues on how to control the life cycles of a broad family of phages, which can lead to important biotechnological applications.
Left: bacteriophages of genus Caudovirales and the family Siphoviridae, similars to SPbeta. Image: Laura Miguel Romero.
Right: structure of SPbeta AimR bound to AimP.
Reference: Francisca Gallego del Sol, José R. Penadés, Alberto Marina. Deciphering the Molecular Mechanism Underpinning Phage Arbitrium Communication Systems. Molecular Cell. DOI: https://doi.org/10.1016/j.molcel.2019.01.025
Source: CSIC Comunicación




