The main aim was to understand the reactivity of biological molecules in contact with metal surfaces. The experiment was fully performed inside the NAPPUHV chambers (sample cleanness, molecular deposition and surface analysis at different pressures).
The adsorption and self-assembly of biological species on solid surfaces can provide important information to understand basic biological reactions,such as one of the most important reaction in living organisms: breathing. This process consists on the transport and storage of the dioxygen/carbon dioxide molecules thanks to haemoglobin formed by Fetetrapyrrole molecules. In order to mimic this process at CIRCE beamline we exposed Fe-phthalocyanine molecules, which had been previously evaporated in vacuum on a clean Au(111) surface, to different O2 partial pressures. By means of a combination of XPS and NEXAFS we detected the anchorage of O2 when pressure in the chamber was changed from UHV to around 1x10-2 mbar, being this process reversible. Thus, when the O2 partial pressure was reduced again O2 liberation took place.
This reversibility was basically detected by analyzing the O 1s core level photoelectron spectra and the Fe2p absorption edge of the system underdifferent pressure conditions inside the chamber. When pressure was around 10-2 mbar, a new component appeared in the O 1s core level region in the XPS spectrum. This new component was completely separated from the O2 in the gas phase, disappeared when the gas was removed and was not observed for the clean Au(111) under the same pressure conditions. Such behaviour indicates that this feature is related with the O2 molecules bonded to the Fe ions in the FePc molecules. Moreover,Fe 2p absorption edge reveals that Fe(II) ions change their conformation during O2 bonding. The central metal core of the phthalocyanine moved from the initial planar configuration to a more tetrahedral structure when the O2 was temporarily bonded to the surface. This change is again reversible, i.e. the system returns to the flat configuration when the gas is removed. Similar conformational canges have been reported in living organisms for the haemoglobin during the breathing process. Thus with this system we confirm that it is possible to mimic these biological processes at the nanoscale.
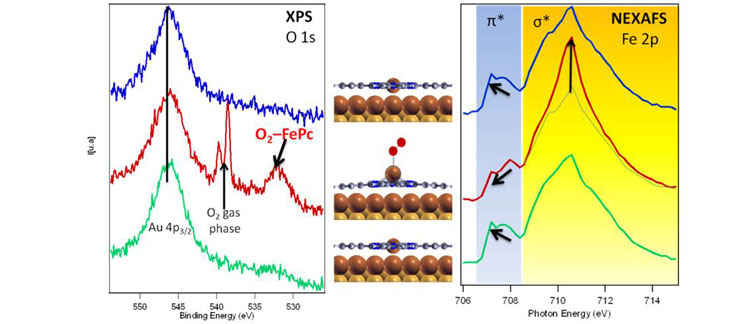
Fig. O1s XPS spectra (left) and Fe 2p absorption edges (right) of FePc/Au(111) measured at a base pressure of 7x10-10mbars (blue spectrum), during O2 partial pressure of 10-2mbars (red spectrum) and after removing the O2, measured at pressure of 1x10-9mbars (green spectrum). Schemes illustrate the conformation of the molecules due to the bond between Fe ions and O2 molecules.
Reference: Celia Rogero, Jorge Lobo-Checa and Mikel Abadía from the Centro de Física de Materiales(CSIC-UPV/EHU) and Donostia International Physics Center




