
Left: Molecular mimicry in the specific recognition of thrombin by TTI. Right: Bárbara Calisto at the XALOC beamline of the ALBA Synchrotron.
Cerdanyola del Vallès, 2nd December 2020 In the waiting rooms of health care facilities around the world, millions of patients take anticoagulants every day. These are life-saving drugs for the treatment of cardiovascular diseases, which now are also being explored for their benefits to patients with advanced symptoms of COVID-19.
And, as incredible as it may seem, the tsetse fly, responsible for the sleeping sickness disease in humans, is now on the spotlight in the efforts to develop more powerful and safer anticoagulants.
In a study co-authored by Bárbara Calisto, researcher at the ALBA Synchrotron, an international team of scientists has become the first to recreate in the lab the molecule that the tsetse fly uses to prevent coagulation when it bites to feed. These bites are also the entry channel for the parasite that causes sleeping sickness, a life-threatening disorder, if untreated. And the reason why the tsetse fly has been dubbed as the fly of death in Africa.
In the study, published in Cell Chemical Biology, researchers also unraveled the unique binding of this molecule to thrombin, a central protein for the cascade of events that causes coagulation in humans and other mammals.
Ticks and mosquitoes, and in general all hematophagous invertebrates –which feed on blood– are known to have evolved exquisite mechanisms to prevent coagulation. But, the tsetse fly has mastered the technique and uses an extremely powerful inhibitor, which for a while scientists were not able to mimic in the lab.
“The molecules previously developed in the lab were 2,000-fold less potent than those isolated from tsetse’s salivary glands. We predicted that modifications happening after the initial natural synthesis could be responsible for the increased anticoagulant efficiency,” explains Bárbara Calisto, from the XALOC beamline at ALBA.
And they were right.
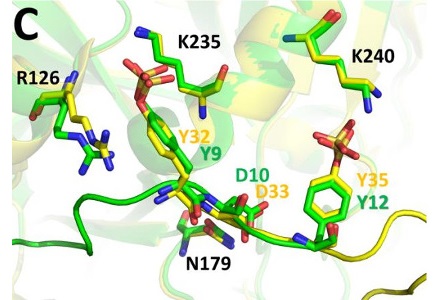
The tsetse natural anticoagulant is a peptide, a molecule similar to proteins but with few amino acids. What Calisto and collaborators suggested was that a chemical modification to specific amino acids in the peptide was at the origin of the increased affinity for thrombin in the native anticoagulant. And, once that modification was also applied to the synthetic peptide, the binding to thrombin increased by three orders of magnitude, thus matching the anticoagulant potency of tsetse bites.
However, new drugs need to address two major concerns: the interaction with food and other drugs, which may require recurring blood tests to adjust dosage, and the management of bleeding, which becomes the source of severe complications. The good news is that the specific binding of the tsetse anticoagulant to thrombin shows very promising features for the development of safer and more stable drugs.
“The more we know about the tsetse anticoagulant, the more convinced we are of its potential for the development of new drugs, namely for use in specific medical settings,” says Calisto.
The X-ray diffraction analyses performed at two synchrotron facilities, the ALBA Synchrotron near Barcelona and the European Synchrotron Radiation Facility (ESRF) in Grenoble, allowed a detailed study of the structure of the molecule and revealed the role of two amino acids as the drivers of its potent binding to thrombin.
“For a long time, we had known about the devastating power of a tsetse bite. Now, thanks to synchrotron light we understand it. Our findings cannot prevent the spread of sleeping sickness, but can be used to develop new anticoagulant drugs to stop the no less devastating cardiovascular diseases, saving many lives. Isn’t that amazing!?,” adds Calisto.
These results anticipate a new generation of drugs which may be less disruptive of the natural management of hemorrhages. They are the outcome of a multidisciplinary approach involving scientists spread around the world, from two major synchrotron research facilities in Spain and France to the teams of Prof. Richard Payne in Australia and Dr. Pedro Pereira in Portugal, who led this study.
Referencia: Sulfotyrosine-Mediated Recognition of Human Thrombin by a Tsetse Fly Anticoagulant Mimics Physiological Substrates, Bárbara M. Calisto, Jorge Ripoll-Rozada, Luke J. Dowman, Charlotte Franck, Stijn M. Agten, Benjamin L. Parker, Rita Carvalho Veloso, Nuno Vale, Paula Gomes, Daniele de Sanctis, Richard J. Payne, Pedro José Barbosa Pereira. Cell Chemical Biology. https://doi.org/10.1016/j.chembiol.2020.10.002




