|
|
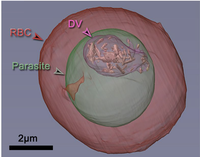
Cerdanyola del Vallès, 4th October 2017. Plasmodium falciparum causes the malaria disease. This parasite, transmitted through mosquito sting, infects red blood cells of its victim. Once inside, it uses hemoglobin (the protein in charge of oxygen transport) as a nutrient. When it is digested, iron is released in a form of heme molecules. These heme molecules are toxic to the parasite, but it has a strategy to make them harmless: it packs heme in pairs and finally they are packed forming hemozoin crystals. In this way, poisonous iron is locked up and no longer will be a threat for the parasite. Tomography of a red blood cell (RBC) with a malaria parasite inside (in green). The parasite has digestive vacuole (DV) where the hemozoin crystals are concentrated. |
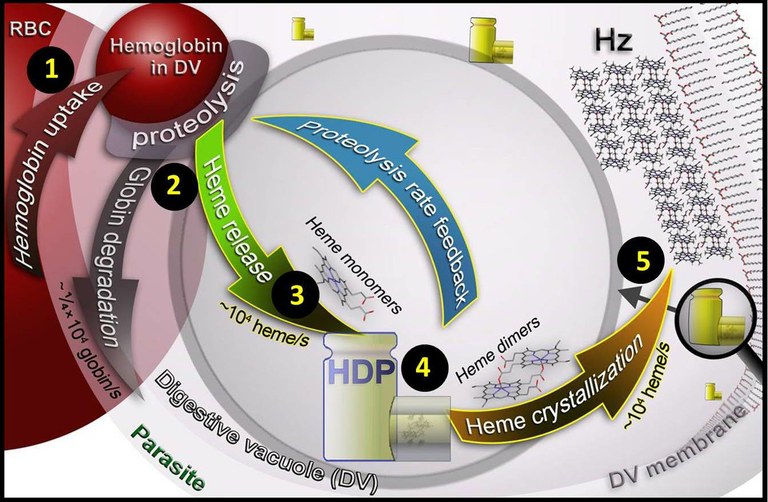
It is still not well understood how the parasite crystalizes heme, however, the research group, headed by Sergey Kapishnikov from University of Copenhagen, has been able to measure the crystallization rate for the first time in frozen snapshots of the in vivo state of the parasite. The key fact in the parasite's process, which occurs inside the red blood cells, is that the two reactions – hemoglobin degradation and heme crystallization – must be coordinated. Like an assembly line, the initial digestion cannot be quicker than the crystallization. If not, toxic heme groups released would be accumulated and the equipment in charge of picking them would not be able to get rid of all heme on time. As a consequence, researchers suggest a new process model where they state the need of a control system which regulates the speed at which the malaria parasite is digesting hemoglobin and thereby also limit the rate of heme release.
This discovery is absolutely useful for new drugs development, whose target could be the sabotage of the parasite's waste management mechanism: either derailing toxic heme's packaging, or causing their accumulation by attacking the coordination system between degradation and crystallization; ending up in the parasite choking on their own litter.
|
|
Model for biochemistry processes that occur inside the parasite. The parasite takes the hemoglobin from the red blood cell (RBC) 1 and digests it inside the digestive vacuole (DV) 2. As a consequence, heme groups are released 3 and HDP protein packages them in pairs (heme dimers) 4. Finally, in the crystallization process these dimers are converted in hemozoin crystals 5. Blue arrow points out the suggested feedback mechanism that regulates hemoglobin degradation. |
The study comes about thanks to the cooperation between international research centres: the Niels Bohr Institute from University of Copenhagen, Helmholtz Research Center from Berlin, Paul Scherrer Institute from Switzerland, Weizmann Institute of Science from Israel, and ALBA Synchrotron, BESSY-II, the Swiss Light Source and the European synchrotron ESRF in France.
Combining two state-of-the-art synchrotron light techniques
In the lab, human red blood cells were infected with Plasmodium falciparum and scientists were able to obtain images in situ developing a new methodology. Its new aspect is that it combines two modern techniques: soft-X-ray microscopy and X-rays fluorescence microscopy. The first one, X-rays microscopy from MISTRAL beamline in ALBA Synchrotron, provides tomographies: high resolution 3D cells images. The first infected red blood cell was studied in Berlin at BESSY-II in U41-XM beamline with the same technique as well. Secondly, X-rays fluorescence microscopy – performed in microXAS beamline from Swiss Light Source and more recently in ID16A-NI beamline of ESRF synchrotron – allows mapping of elements such as iron, potassium and sulphur in different parts of the cells.
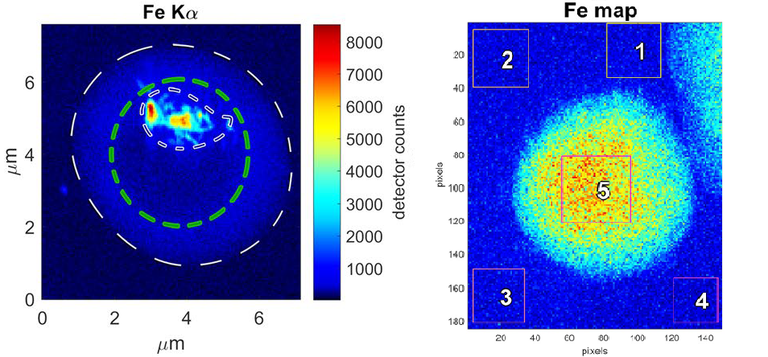
The ratio of iron and sulphur is interesting, for example, so it can be guessed whether the iron detected with fluorescence comes from hemozoin crystals, hemoblogin or released heme; enabling to calculate the concentrations of all these molecules. Moreover, analysing the amount of iron inside the parasite as a function of time, the crystallization rate has been deduced. Since the different models proposed for heme crystallization predict vastly different rates, one way to identify the crystallization mechanism is to measure this rate in vivo.
|
|
Iron fluorescence maps. (Left) Infected red blood cell where the iron is inside the digestive vacuole. This iron corresponds to hemozoin crystals. Iron distribution is clearly different from pristine red blood cell (right), where iron atoms are for the whole cytosol as hemoglobin molecules. One pixel is 50nm. |
Finally, the analysis of microscope images provides also the first measurement of non-crystallized heme present inside the parasite. This is indeed remarkable due to, considering the calculated rate, processing this amount of heme will spend 1.4 hours and it is vastly known these molecules are immediately toxic. That fact means the parasite is somehow able to block hemo to make them harmless: "probably associating heme with hemoglobin not yet digested" suggest the researchers.
A new target against the parasite
All of these results yield the research group to propose a new crystallization model with a feedback mechanism, since the two steps must be coordinated: hemoglobin digestion cannot be quicker than crystallization. Not knowing exactly the parasite processes has hampered the development of new drugs against malaria, a disease that affects hundreds of million people all over the world, the most part of them children. Nowadays, resistances which appear after some time using a specific drug claim the must of new more efficient treatments that could target heme detoxification. So, next step is discover how exactly crystallization and hemoglobin degradation are coordinated in order to find out a new target. If this information mechanism is interrupted, the parasite would die.