Cerdanyola del Vallès, 31st October 2018 Researchers from the Molecular Biology Institute of Barcelona (IBMB-CSIC) and the Institute of Biotechnology and Biomedicine (IBB-UAB) have discovered the mechanism by which the bacterium Mycoplasma genitalium (Mgen) adheres to human cells. This adhesion is essential for the onset of bacterial infection and subsequent disease development.
Mgen is an emerging pathogen responsible for several infectious genitourinary disorders. In men, it is the most common cause of urethritis (15-20%) while in women, it has been associated with cervicitis, pelvic inflammatory disease, premature birth and spontaneous abortions. So far, it was known that adherence to the genitourinary tract was possible thanks to proteins known as adhesins, which recognise specific cell surface receptors.
In this study, IBMB-CSIC researchers determined the three-dimensional structure of the Mgen's P110 adhesins interacting with these cell receptors using X-rays diffraction and protein crystallography at the XALOC beamline. "We made a protein crystal of the P110 adhesin bound to these receptors and diffracted with the synchrotron’s X-rays to determine the exact position of the atoms within the protein, and we were able to decipher the three-dimensional structure", explains IBMB researcher David Aparicio.
At the same time, IBB-UAB scientists conducted in vivo studies with human cells and demonstrated that mutations in specific sites of the P110 protein prevent the adherence of Mgen. These results were fundamental to confirm the information obtained from the three-dimensional structure.
The results allow a better understanding of the molecular bases of the Mgen interaction with human cells. "On the one hand, we have obtained key information on the process of colonisation, that is how the pathogen comes into contact with the host cells. On the other hand, it allows us to develop alternative drugs capable of blocking Mgen's cell adhesion, such as molecules mimicking the human cell receptors, or stimulating the formation of antibodies which can inhibit the function of these adhesins", explains IBB research Oscar Quijada.
The research has led to an international patent application and a new collaboration with the Microbiology Department and research group from the Vall d'Hebron Campus with the aim of fighting against the emergence of new resistances.
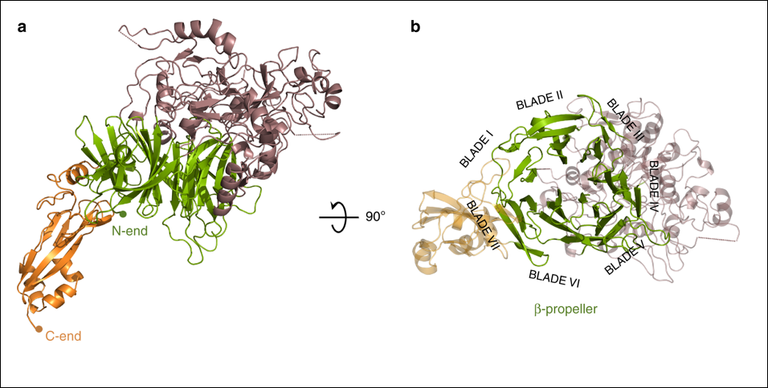
Overall structure of P110. Two views, 90° apart from each other, of the extracellular region of P110 that is formed by a large N-domain, with a seven blade β-propeller (green), the crown (brown), and the C-domain (orange). In the right side panel the view is along the central axis of the β-propeller. The situation of the seven blades in the propeller is explicitly indicated showing that the two terminal blades I and VII are close to the C-terminal domain and opposite to the crown.
Antibiotic Resistance
Currently, Mgen infections are as frequent as gonorrhoea infections, one of the most common sexually transmitted diseases. In addition, Mgen is becoming a superbug capable of resisting all available antibiotics, which will soon leave humans with no alternative therapies to fight infections. Antibiotic resistance is rising to dangerously high levels. Through genetic changes, many bacteria have developed the capacity to become resistant to antibiotics and continue to reproduce themselves. Although this is a natural process, inadequate use and abuse of these drugs are accelerating the process. Given that Mgen is becoming resistant to all available antibiotics, finding an alternative therapeutic strategy is of utmost importance. The results obtained are essential for the design of new drugs thanks to the ability to define adhesion at molecular level.
Reference: David Aparicio, Sergi Torres-Puig, Mercè Ratera, Enrique Querol, Jaume Piñol, Oscar Q. Pich, and Ignacio Fita. Mycoplasma genitalium adhesin P110 binds sialic-acid human receptors. Nature Communications. DOI 10.1038 / s41467-018-06963-i
Original new at Universitat Autònoma de Barcelona website




