Cerdanyola del Vallès, 15th October 2019 MOFs (metal-organic frameworks) are porous materials with well-defined geometry and high loading capacity. For biological applications, their high porosity makes these composites an effective strategy for loading and protection of proteins; however, their use for other biomacromolecules such as nucleic acids is still in their infancy. Now, a research team lead by RMIT University from Melbourne has been studying the use of ZIF-8 MOFs as possible gene delivery vectors. The results show encapsulation of a gene-set in ZIF-8 MOFs and its cellular expression, proving that MOFs do not damage the structural and functional activity of the cargo nucleic acid, essential for possible applications in gene therapy as disease treatments.
Scientists have used a plasmid (DNA molecule that can express proteins) which carries genes that code for a green fluorescent protein (GFP). This plasmid (plGFP) is routinely used for molecular biology studies and here it works as a proof-of-concept genetic macromolecule. ZIF-8 encapsulated plGFP (plGFP@ZIF-8) has been introduced into prostate cancer epithelial cells. plGFP itself is non-fluorescent, but when transcribed and translated into GFP protein, the cell emits a strong green fluorescence. This enables researchers to check if the DNA plasmid has been transfected and expressed correctly inside the cell or not.
Intracytoplasmic and endocytic presence of the plGFP@ZIF-8 particles were verified and visualized using correlative cryo-epifluorescence microscopy and soft X-ray cryo-tomography at MISTRAL beamline. This microscope located at the ALBA Synchrotron is the only modality that allows imaging of whole cell samples in their native state without any chemical treatment or sectioning. Gradual protein expression as a green fluorescence in the cells could be recorded, proving the ability of MOFs to maintain the chemical and physical integrity of DNA by conserving its function.
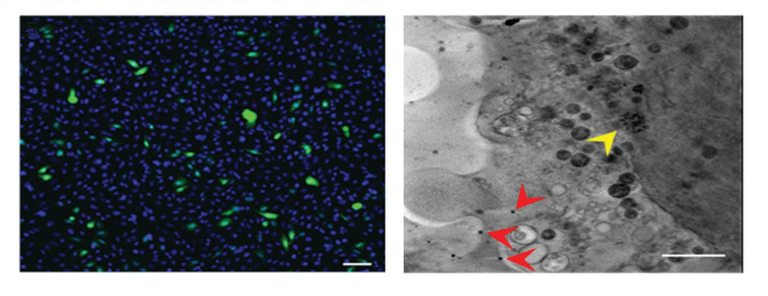
Left: Confocal laser scanning microscope images of plGFP@ZIF-8 transfected into human prostate cancer epithelial cells. Right: Cryo Soft X-ray tomogram section, the yellow arrow head points to an endocytic organelle containing densities compatible with ZIF-8 MOFs and the red arrow heads point to high intracytoplasmic densities compatible with ZIF-8 MOFs.
The retention of functional activity, intracellular delivery, and expression of encapsulated genes occurred over relatively prolonged time periods without apparent cytotoxicity. This has huge potential in the field of gene therapy where the delivery system itself should be nontoxic and in cases where a sustained expression over time is required instead of burst release of therapeutic load. It is expected that this approach will result in a promising gene delivery agent that protects and maintains functional activity of DNA and affords customized expression of genes leading to specifically designed persistence of therapeutic levels in vivo.
Gene therapy in disease treatment
Gene therapy holds great promise in disease treatment; however, the delivery of therapeutic genes is a severe impediment in the overall success of this approach. Nowadays scientists are looking for good delivery vectors. Over 70% of the gene therapy clinical trials involve viral vectors and only about 19% of trials utilize non-viral based delivery systems. Although viral therapy is effective and powerful for some diseases, nonviral delivery systems are safer and less immunogenic, relatively inexpensive to produce and have a large DNA carrying capacity. However, there are still significant challenges to deal with: maintaining the chemical and physical integrity of DNA; successful entry through the cell membrane; and endosomal escape prior to nuclear uptake for expression of the gene. Therefore, the development of improved synthetic systems that allow the vector to address the above limitations is essential and this study reports the potential of MOFs as suitable gene therapy systems for DNA delivery.
Reference: A. Poddar, J.J. Conesa, K. Liang, S. Dhakal, P. Reineck, G. Bryant, E. Pereiro, R. Ricco, H. Amenitsch, C. Doonan, X. Mulet, C. M. Doherty, P. Falcaro, R. Shukla. Encapsulation, Visualization and Expression of Genes with Biomimetically Mineralized Zeolitic Imidazolate Framework-8 (ZIF-8). Small (2019) 1902268. DOI:10.1002/smll.201902268




