Cerdanyola del Vallès, 17th May 2019 Researchers from Centro de Investigación del Cáncer - Instituto de Biología Molecular y Celular del Cáncer of Salamanca, from Centro Universitario de la Defensa in Zaragoza, and from the Netherlands Cancer Institute in Amsterdam have described how two essential proteins interact to each other in order to join epidermis and dermis together. This study reveals at atomic scale how the binding between two hemidesmosomal proteins called integrin α6β4 and BP230 takes place.
Epithelial tissues, such as epidermis, settle on fibrous sheets called basement membrane, formed by extracellular matrix proteins. The junction between epithelia and basement membrane happens through hemidesmosomes, multi-protein complexes located at the membrane of epithelial cells. Integrin α6β4 is an essential protein of the hemidesmosomes, which adheres to proteins of the basement membrane. Inside the cell cytoplasm, plectin and BP230 proteins bind to α6β4 and connect it to the intermediate filaments of the cytoskeleton. Genetic or autoimmune alterations that affect the hemidesmosomal proteins reduce skin resistance and cause diseases such as bullous pemphigoid and various types of epidermolysis bullosa.
"Despite the relevance of hemidesmosomes for the integrity of the skin and other epithelial tissues, little is known about the structure and the organization of these complexes. Nor it is clear how these structures are formed and disassembled," explains José María de Pereda, CSIC researcher in the Instituto de Biología Molecular y Celular del Cáncer and lead scientist of the group.
In order to understand how hemidesmosomes are organized, researchers have analyzed the interaction between the α6β4 and BP230. Firstly, the α6β4 and BP230 regions where takes place the contact have been identified. After that, scientists have used X-ray crystallography to solve the structure of β4 bound to BP230. "Beforehand, we had characterized the interaction between α6β4 and plectin, and now, we have seen that BP230 binds α6β4 in a totally different way," explains Dr. de Pereda. This analysis has been carried out in the macromolecular diffraction beamline XALOC, at the ALBA Synchrotron, which allows solving protein structures at atomic level. "The high resolution of this technique has enabled us to identify some amino acids in α6β4 and BP230 that play a key role for both proteins to fit," he adds. Scientists have also performed experiments in other synchrotrons like Diamond (United Kingdom) and EMBL-DESY (Germany).
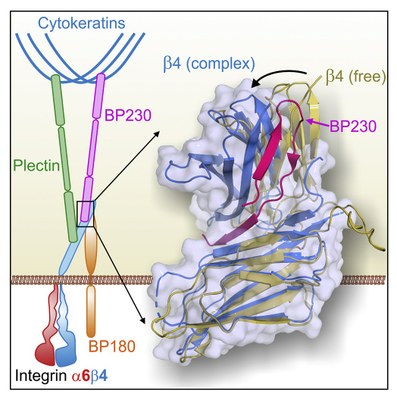
Structure of β4(WT)-BP230 complex.
The research has also unveiled that the binding of BP230 to α6β4 is necessary for the incorporation of BP230 into hemidesmosomes. On the other hand, the results suggest some mechanisms that could break this union when the hemidesmosomes dissociate, for example during wound healing.
Reference: Manso JA, Gómez-Hernández M, Carabias A, Alonso-García N, García-Rubio I, Kreft M, Sonnenberg A, de Pereda JM (2019). Integrin α6β4 recognition of a linear motif of bullous pemphigoid antigen BP230 controls its recruitment to hemidesmosomes. Structure 27, 1-13.




